Allergic Asthma - Sullivan A, Kushnir NM (Updated 2020)
Allergic Asthma: Symptoms and Treatment
Updated: October 2020
July 2015
Posted: May 2006
Updated by:

Ashley A. Sullivan, MSN FNP
Student, Samuel Merritt University, Oakland, Ca
RN, California Pacific Medical Center

Natalya M. Kushnir, MD
Director, Allergy and immunology Clinic of East Bay
Berkeley, CA
Original authors:
H. Henry Li, MD, PhD
FAAAAI, FACAAI
Institute for Asthma and Allergy
Wheaton and Chevy Chase Maryland
Michael A. Kaliner, MD FAAAAI
Medical Director, Institute for Asthma and Allergy
Chevy Chase and Wheaton, Maryland
Professor of Medicine, George Washington University School of Medicine
Washington DC
Definition and demographics
Asthma is truly a syndrome encompassing several disease entities/endotypes. The word asthma derives from the Greek word for panting, or breathlessness, and thus describes the primary symptom of this disease. Asthma is recognized as a complex condition with differences in severity, natural history, comorbidities, and treatment response. It has been defined as "a chronic inflammatory disorder associated with variable airflow obstruction and bronchial hyperresponsiveness. It presents with recurrent episodes of wheeze, cough, shortness of breath, chest tightness."
While the critical role of inflammation has been further substantiated, there is an evidence for considerable variability in the pattern of inflammation indicating phenotypic differences that may influence treatment responses. Gene-by-environmental interactions are important to the development and expression of asthma. Of the environmental factors, allergic reactions and pollution are of critical importance with expanding role for viral respiratory infections in these processes. The onset of asthma for most patients begins early in life with the pattern of disease persistence determined by early, recognizable risk factors including atopic disease, recurrent wheezing, and a parental history of asthma. Current asthma treatment with anti-inflammatory does not appear to prevent progression of the underlying disease severity.
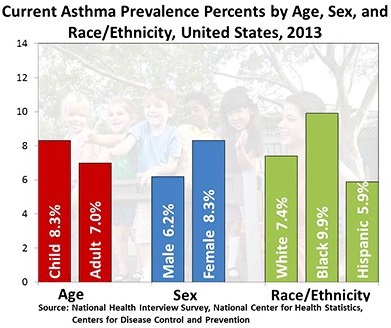
Asthma is the most common chronic respiratory disorders, affecting all age groups, worldwide. The most recent comprehensive analyses of the Global Burden of Disease Study (GBD) undertaken in 2008-2010 estimates the number of people with asthma in the world as high as 334 million. A lower figure of 235 million used in the Global Asthma Report 2011 came from the most up to date GBD information available at that time based on analyses from 2000-2002. Prevalence of childhood asthma varies widely between countries, and between centers within countries, and estimated at 14%. Prevalence of recent wheeze in adolescents varied widely. The highest prevalence (>20%) was generally observed in Latin America and in English-speaking countries of Australasia, Europe and North America as well as South Africa. The lowest prevalence (<5%) was observed in the Indian subcontinent, Asia-Pacific, Eastern Mediterranean, and Northern and Eastern Europe. In Africa, 10-20% prevalence was mostly observed. Overall, 4.3% of respondents to the World Health Survey aged 18-45 in 2002-2003 reported a doctor’s diagnosis of asthma, 4.5% had reported either a doctor’s diagnosis or that they were taking treatment for asthma, and 8.6% reported that they had experienced attacks of wheezing or whistling breath (symptoms of asthma) in the preceding 12 months.
Prevalence of asthma in middle-aged and older American adults is found to be higher in women ( 9.7%) and higher among adults who are poor (10.6%). There is greater difficulty of distinguishing asthma from other respiratory conditions, such as chronic obstructive pulmonary disease (COPD) in older age groups. Geriatric asthma can be complicated by comorbidities, potential loss of reversibility of airway obstruction, as well as impairment in the perception of breathlessness. Limited data remains in clinical trials on geriatric asthma as asthma medications are rarely tested on the elderly. Due to asthma being commonly thought of as a childhood disease, the elderly are often under-diagnosed and undertreated.
Clinical Classification
It is increasingly clear that asthma syndrome is divided into distinct disease entities with specific mechanisms. The attempt for a new classification is made were "endotype" is proposed to be a subtype of a condition defined by a distinct pathophysiological mechanism. Criteria for defining asthma endotypes on the basis of their phenotypes and putative pathophysiology are suggested.
Currently asthma is classified into atopic and non-atopic types based on the onset of symptoms. Atopic refers to early-onset whereas non-atopic refers to late-onset. Despite the differentiation, a significant degree of overlap exists between the two types. The severity of symptoms is further classified based on the GINA severity grades into mild intermittent, mild persistent, moderate persistent and severe persistent asthma. Furthermore, asthma severity classification is different for various ages.
Signs and Symptoms of Asthma
To establish a diagnosis of asthma, the clinician should determine that:
- Episodic symptoms of airflow obstruction or airway hyperresponsiveness are present.
- Airflow obstruction is at least partially reversible.
- Alternative diagnoses are excluded.
Recommended methods to establish the diagnosis are:
- Detailed medical history.
- Physical exam focusing on the upper respiratory tract, chest, and skin.
- Spirometry to demonstrate obstruction and assess reversibility, including in children 5 years of age or older. Reversibility is determined either by an increase in FEV1 of ≥12 percent from baseline or by an increase ≥10 percent of predicted FEV1 after inhalation of a short-acting bronchodilator.
Additional studies are not routinely necessary but may be useful when considering alternative diagnoses:
- Additional pulmonary function studies (e.g., measurement of lung volumes and evaluation of inspiratory loops) may be indicated, especially if there are questions about possible coexisting COPD, a restrictive defect, VCD, or possible central airway obstruction. A diffusing capacity test is helpful in differentiating between asthma and emphysema in patients, such as smokers and older patients, who are at risk for both illnesses.
- Bronchoprovocation with methacholine, histamine, cold air, or exercise challenge may be useful when asthma is suspected, and spirometry is normal or near normal. For safety
- reasons, bronchoprovocation testing should be carried out by a trained individual in an
- Appropriate facility and is not generally recommended if the FEV1 is <65 percent predicted. A positive methacholine bronchoprovocation test is diagnostic for the presence of airway hyperresponsiveness, a characteristic feature of asthma that also can be present in other conditions (e.g., allergic rhinitis, cystic fibrosis, COPD, among others). Thus, although a positive test is consistent with asthma, a negative bronchoprovocation may be more helpful to rule out asthma.
- Chest x ray may be needed to exclude other diagnoses.
- Allergy testing
- Biomarkers of inflammation. The usefulness of measurements of biomarkers of inflammation (e.g., total and differential cell count and mediator assays) in sputum, blood, urine, and exhaled air as aids to the diagnosis and assessment of asthma
It is important to consider a diagnosis of asthma if certain elements of the clinical history are present – they are not diagnostic by themselves but increase the probability of a diagnosis of asthma:
- Wheezing—high-pitched whistling sounds when breathing out—especially in children. (Lack of wheezing and a normal chest examination do not exclude asthma.)
- History of any of the following:
- Cough, worse particularly at night
- Recurrent wheeze
- Recurrent difficulty in breathing
- Recurrent chest tightness
- Symptoms occur or worsen in the presence of:
- Exercise
- Viral infection
- Animals with fur or hair
- House-dust mites (in mattresses, pillows, upholstered furniture, carpets)
- Mold
- Smoke (tobacco, wood)
- Pollen
- Changes in weather
- Strong emotional expression (laughing or crying hard)
- Airborne chemicals or dusts
- Menstrual cycles
- Symptoms occur or worsen at night, awakening the patient.
Spirometry is needed to establish a diagnosis of asthma.
Physical examination should be focused on upper respiratory tract, chest, and skin. Certain findings present on physical exam increase the probability of asthma, while their absence does not rule it out, because the disease is by definition variable, and signs of airflow obstruction are often absent between attacks:
- Hyper expansion of the thorax, especially in children; use of accessory muscles; appearance of hunched shoulders; and chest deformity.
- Sounds of wheezing during normal breathing, or a prolonged phase of forced exhalation (typical of airflow obstruction). Wheezing may only be heard during forced exhalation, but it is not a reliable indicator of airflow limitation.
- Increased nasal secretion, mucosal swelling, and/or nasal polyps.
- Atopic dermatitis/eczema or any other manifestation of an allergic skin condition.
The presence of concomitant diseases or conditions that may influence asthma, including:
- Rhinosinusitis,
- Gastro-esophageal reflux or laryngopharyngeal reflux, and
- Bronchitis or smoking.
Early in the disease, symptoms may include a vague, heavy feeling of tightness in the chest and in the allergic patient, there may be associated rhinitis and conjunctivitis symptoms. Typical symptoms which patients experience include coughing, wheezing, chest tightness and dyspnea. Cough in asthma is usually non-productive, but it may progress to expectoration of viscous, mucoid sputum which is difficult to clear. If the sputum turns purulent or discolored, an infection may be present, as the sputum in asthma is usually clear to light yellow in color.
There is a subgroup of asthmatics whose asthma is characterized solely by cough, without overt wheezing, the "cough variant of asthma". Monitoring of PEF or methacholine inhalation challenge, to clarify whether there is bronchial hyperresponsiveness consistent with asthma, may be helpful in diagnosis. The diagnosis of cough variant asthma is confirmed by a positive response to asthma medication.
In the completely asymptomatic patient, results of chest examination will be normal, although head, eye, ear, nose, and throat examination may disclose concomitant serous otitis media, allergic conjunctivitis, allergic rhinitis, nasal polyps, paranasal sinus tenderness, signs of postnasal drip, or pharyngeal mucosal lymphoid hyperplasia. Clubbing of the fingers is extremely rare in uncomplicated asthma, and this finding should direct the physician's attention toward diseases such as bronchiectasis, cystic fibrosis, pulmonary neoplasm, or cardiac disease. Many symptomatic asthmatics can be diagnosed by careful auscultation of the chest which reveals the presence of expiratory wheezing and a somewhat prolonged expiratory phase.
Exacerbations of asthma are acute or subacute episodes of progressively worsening shortness of breath, cough, wheezing, and chest tightness—or some combination of these symptoms. Exacerbations are characterized by decreases in expiratory airflow that can be documented and quantified by simple measurement of lung function (spirometry or PEF), can vary widely among individuals and within individuals from rare to frequent. It is important to understand that the severity of disease does not necessarily correlate with the intensity of exacerbations, which can vary from mild to very severe and life-threatening.
Patients at any level of severity, even intermittent asthma, can have severe exacerbations. For example, a person who has intermittent asthma can have a severe exacerbation during a viral illness or when exposed to allergens to which he or she is sensitized or to noxious fumes and irritants. In fact, the last classification “mild intermittent asthma” was changed to “intermittent asthma”, emphasizing that patients at any level of severity — including intermittent — can have severe exacerbations. The frequency of exacerbations requiring intervention with oral systemic corticosteroids now changed to classification of persistent, rather than intermittent asthma. However, severity can determine prolongation of the illness and is often characterized by unremitting symptoms with poor response to therapy. The duration of acute exacerbations may vary from a few hours to a few days. These unpredictable variations in exacerbations can present treatment dilemmas in clinical practice.
Assessment of severity requires assessing the following components of current impairment:
- Symptoms
- Nighttime awakenings
- Need for SABA for quick relief of symptoms
- Work/school days missed
- Ability to engage in normal daily activities or in desired activities
- Quality-of-life assessments
- Lung function, measured by spirometry: FEV1, FVC (or FEV6), FEV1/FVC (or FEV6 in adults). Spirometry is the preferred method for measuring lung function to classify severity. Peak flow has not been found to be a reliable variable for classifying severity.
Assessment of Risk
Assessment of the risk of future adverse events requires careful medical history, observation, and clinician judgment. Documentation of warning signs and adverse events will be necessary when a patient is felt to be at increased risk. Patients who are deemed at increased risk of adverse outcomes need close monitoring and frequent assessment by their clinicians.
Predictors that have been reported to be associated with increased risk of exacerbations or death include:
- Severe airflow obstruction, as detected by spirometry
- Persistent severe airflow obstruction. Two or more ED visits or hospitalizations for asthma in the past year; any history of intubation or ICU admission, especially if in the past 5 years
- Patients report that they feel in danger or frightened by their asthma
- Certain demographic or patient characteristics: female, nonwhite, nonuse of ICS therapy, and current smoking
- Psychosocial factors: depression, increased stress, socioeconomic factors
- Attitudes and beliefs about taking medications
Asthma in elderly
Asthma affecting individuals across the lifespan. Current evidence consistently suggests that asthma is common among elderly subjects. Because of increased longevity, the proportion of individuals aged 65 years and older is increasing worldwide. By 2030, elderly subjects will comprise ~20% and ~36% of the populations of the United States (U.S.) and China, respectively. Determining the exact prevalence of asthma in elderly is made difficult by under-diagnosis due to decreased perception or under-reporting of symptoms by patients, suboptimal utilization of spirometry, misclassification of asthma as chronic obstructive pulmonary disease (COPD), and failure to recognize asthma in subjects with co-morbidities such as congestive heart failure or COPD. In two nationwide surveys in the U.S. estimates of the prevalence of current asthma in the elderly were 5.9% for the period 1980–2004. In elderly subjects, asthma is more common in women than in men. Compared to children or younger adults, older adults and/or elderly subjects have greater morbidity and healthcare costs from asthma, thus it is important to recognize and treat asthma in older population.
Causes of Asthma
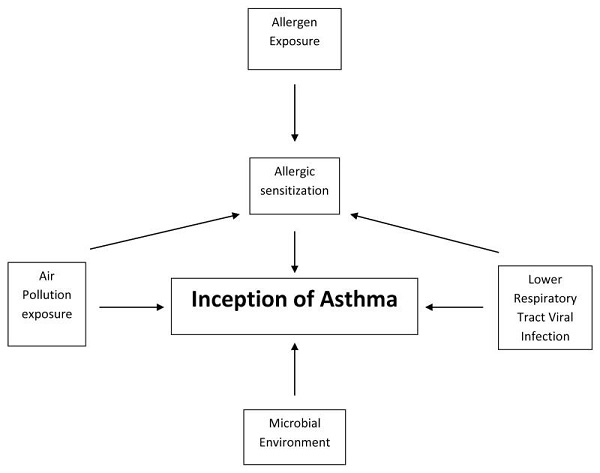
The allergic asthma phenotype dominates in early life. Although asthma has a strong genetic component, environmental factors must occur for it to manifest. The paradigm for allergen induction of asthma is from allergen exposure → allergic sensitization → asthma development. While a variety of ambient and indoor allergic exposures have been implicated in the development and exacerbation of childhood asthma, the indoor environment has greatest influence on asthma development. Children sensitized to aeroallergens at a young age are likely to have persistent asthma symptoms into late childhood and adulthood and show poorer lung function than those not sensitized. House dust mite (HDM), furred pets, cockroach, rodent and mold, with regional variation, account for the large proportion of aeroallergens associated with sensitization and asthma. In many cases, exposure and sensitivity follow a. Evidence supporting dose-response relationship is particularly strong for dust mite and cat.
The steady increase in population trends towards urban centers also shares the trajectory of increasing air pollution. Indoor and ambient air pollution have been associated with a variety of adverse cardiopulmonary health effects including asthma symptoms, exacerbations and decline in lung function. The pollutants best studied are the gases nitrogen dioxide (NO2), ozone (O3), volatile organic compounds (VOCs), and particulate matter (PM) that comprises soot.
Recent evidence has demonstrated elevated pollution exposure in utero and in the first year of life may influence the development of asthma in young children. Exposure to indoor pollution of PM2.5 and VOCs is directly correlated with asthma inflammatory markers in schoolchildren with and without asthma, indicating potential induction of allergic airway inflammation with these exposures.
Environmental tobacco smoke (ETS) is an independent determinant of the development of asthma. Tobacco smoke contains many VOCs and NO2, which are likely to serve as the conduits to poor respiratory outcomes. In vivo studies also suggest that exposure to ETS is associated with IL-13 and greater serum IgE in children with asthma compared to non-exposed asthmatic children and controls, suggesting an augmentation of the Th2 immunophenotype with exposure.
Since the early 2000s the inverse relationship between farming, particularly traditional dairy farming lifestyle, and the development of asthma has been demonstrated early in life and appears to hold true well into adulthood. Children living on farms also had reduced rates of sensitization and other atopic conditions. Farm studies have implicated the rich diversity of microbial exposure both in the animal and home environments are strongly and inversely associated with asthma, implying that the early and persistent microbial environment influences the development of the immune system away from allergic and asthmatic predisposition.
The intestinal microbiome likely influences the immune system in a manner similar to that related to farm exposure.
Because limiting exposure to allergens and allergy immunotherapy are both specifically helpful in treating allergic asthmatic subjects, a careful search for possible allergies is indicated in nearly all asthmatics, certainly all persistent asthmatics.
In addition to allergen-induced asthma, many other factors and conditions such as exercise, infection, occupational chemical exposures, side effects to medications such as beta adrenergic blocking agents, bronchitis, and Churg-Strauss allergic granulomatosis can also cause asthma. Sinusitis, GERD, hyperthyroidism, pregnancy and viral illnesses may complicate asthma.
Pathogenesis and genetics
Over the last decade research has confirmed the important role of inflammation in asthma, unfortunately specific processes related to the transmission of airway inflammation to specific pathophysiologic consequences of airway dysfunction and the clinical manifestations of asthma have yet to be fully understood. Similarly, much has been learned about the host –environment factors that determine airways’ susceptibility to these processes, but the relative contributions of either and the precise interactions between them that leads to the initiation or persistence of disease is difficult to establish. The concepts underlying asthma pathogenesis have evolved dramatically in the past 25 years and are still undergoing evaluation as various phenotypes of this disease are defined and greater insight links clinical features of asthma with genetic patterns.
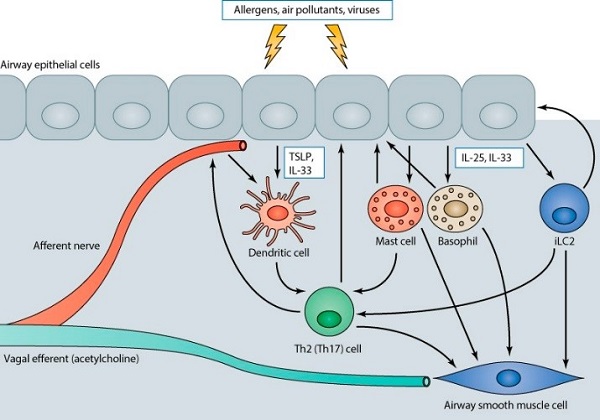
Because asthma involves an integrated response in the conducting airways of the lung to known or unknown triggers, it is a multicellular disease, involving abnormal responses of many different cell types in the lung.
Environmental triggers concurrently act on airway afferent nerves (which both release their own peptide mediators and stimulate reflex release of the bronchoconstrictor acetylcholine) and airway epithelial cells to initiate responses in multiple cell types that contribute to the mucous metaplasia and airway smooth muscle bronchoconstriction that characterize asthma.
Epithelial cells release TSLP and IL-33, which act on airway dendritic cells, and IL-25, which together with IL-33 acts on mast cells, basophils, and innate type 2 lymphocytes. These secreted products stimulate dendritic cell maturation that facilitates the generation of effector T cells and triggers the release of both direct bronchoconstrictors and Th2 cytokines from innate immune cells, which feedback on both the epithelium and airway smooth muscle and further facilitate amplification of airway inflammation through subsequent adaptive T cell responses.
Asthma is genetically heterogeneous. A few common alleles are associated with disease risk at all ages. Implicated genes suggest a role for communication of epithelial damage to the adaptive immune system and activation of airway inflammation. Asthma runs strongly in families, and its heritability has been estimated as 60%. Genetic studies offer a structured means of understanding the causes of asthma as well as identifying targets that can be used to treat the syndrome. Recent genome-wide association studies begun to shed light on both common and distinct pathways that contribute to asthma and allergic diseases. Associations with variation in genes encoding the epithelial cell-derived cytokines, interleukin-33 (IL-33) and thymic stromal lymphopoietin (TSLP), and the IL1RL1 gene encoding the IL-33 receptor, ST2, highlight the central roles for innate immune response pathways that promote the activation and differentiation of T-helper 2 (Th2) cells in the pathogenesis of both asthma and allergic diseases. The factor of atopy, or the genetic tendency for development of the condition, remains the strongest predisposing factor for the development of asthma. These and other genetic findings expanding our understanding of the common and unique biological pathways that are dysregulated in these related conditions and eventually will be helpful in design of new therapies and prevention modalities.
Prevention
Multifactorial disease requires multiple approaches in order to minimize development or progression of the clinical symptoms.
- Environment control
Most convincing evidence for early life environmental exposures influencing the development of asthma would be from randomized controlled interventions to specifically addressing the offending agent and demonstrate lower incidence of asthma development. Allergen remediation strategies directed at cat, dog, mold, mouse and cockroach demonstrate substantially decrease exposure levels in homes. Interventions to reduce HDM alone have been effective and seem to improve early outcomes.
Recent meta-analyses have shown multifaceted allergen remediation programs to be protective against the development of asthma with 20-50% reduction in odds. The most protective effect was seen in children with greater than 5 years of follow-up, indicating a true decrease in risk to those prone to develop atopic asthma.
The best preventative effect of allergen avoidance was the Canadian Childhood Asthma Primary Prevention Study in a high-risk birth cohort. In this study, the intervention was avoidance of house dust mite, pets, and environmental tobacco smoke starting prenatally, and encouragement of breastfeeding with delayed introduction of solids. HDM interventions included encasing parents’ and infants’ mattresses and box springs, weekly hot water wash of all bedding and application of benzyl benzoate to carpets and upholstery before birth and at 4 and 8 months of age. Children receiving the intervention had significantly less physician diagnoses of asthma, wheeze in the past 12 months and wheeze apart from colds when evaluated at age 7 years. Another birth cohort study also observed significantly fewer asthma symptoms at age 8 years old in a high-risk birth cohort intervention focused on HDM and food allergen avoidance in early life, and a significant decrease in atopy at the 8-year time point.
Large studies assessing increased exposure to indoor fungi before the development of asthma symptoms suggests that Penicillium, Aspergillus, and Cladosporium species pose a respiratory health risk in susceptible populations. Increased exacerbation of current asthma symptoms in children and adults were associated with increased levels of Penicillium, Aspergillus, Cladosporium, and Alternaria species, although further work should consider the role of fungal diversity and increased exposure to other fungal species.
- Probiotics and vitamins
Early studies on the effect of probiotics to affect asthma development by influencing the perinatal microbiome have been mixed. A recent study found significant decrease in the risk of atopic sensitization associated with pre and post-natal administration of probiotics, however there was no effect on asthma or wheeze.
Vitamins are essential constituents of our diet that have long been known to influence the immune system. Vitamins A and D have received particular attention in recent years as these vitamins have been shown to have an unexpected and crucial effect on the immune response.
- Experimental preventive therapies
In preterm infants without bronchopulmonary dysplasia, Palivizumab, a monoclonal antibody against RSV, reduces respiratory morbidity up to 78%. Recent findings in late preterm children without BPD suggests that prophylaxis through infancy may decrease recurrent wheeze in the first year of life by 10% and by up to 50% at three years of age. While encouraging, further longitudinal studies are necessary to evaluate the effect of palivizumab prophylaxis to decrease the incidence of asthma in childhood.
Recent evidence suggests that anti-allergen immunotherapy to cross-link the FcέR1 receptor may diminish viral induced asthma symptoms. While alterations of the physical environment have been studied, little attention has been given to the approach of altering the immune constitution of high-risk individuals. In this respect, immunomodulators, such as Omalizumab may be of future interest.
Treatment
Treatment with anti-inflammatory drugs can, to a large extent, reverse some of these processes; however, the successful response to therapy often requires weeks to achieve and, in some situations, may be incomplete.
The goals of asthma treatment include improving quality of life for people who have asthma in addition to controlling symptoms, reducing the risk of exacerbations, and preventing asthma-related death.
A recent large international trial demonstrated that significant reductions in the rate of severe exacerbations and improvements in quality of life were achieved by aiming at achieving guideline-defined asthma control and by adjusting therapy to achieve it. It is important, therefore, to examine how the disease expression and control are affecting the patient’s quality of life. Specific clinical assessment questionnaires were generated to assist practicing physicians in asthma patient evaluation:
Asthma-Specific Quality of Life
- Mini Asthma Quality of Life Questionnaire (Juniper et al. 1999a)
- Asthma Quality of Life Questionnaire (Katz et al. 1999; Marks et al. 1993)
- ITG Asthma Short Form (Bayliss et al. 2000)
- Asthma Quality of Life for Children (Juniper et al. 1996)
Generic Quality of Life
- SF-36 (Bousquet et al. 1994)
- SF-12 (Ware et al. 1996)
The change in emphasis from previous practice guidelines is in periodic assessment of asthma control. For initiating treatment, asthma severity should be classified, and the initial treatment should correspond to the appropriate category of severity. Once treatment is established, the emphasis is on assessing asthma control to determine if the goals for therapy have been met and if adjustments in therapy (step up or step down) would be appropriate.
Components considered essential to effective asthma management:
Measures of assessment and monitoring, obtained by objective tests, physical examination, patient history and patient report, to diagnose and assess the characteristics and severity of asthma and to monitor whether asthma control is achieved and maintained
- Education for a partnership in asthma care
- Control of environmental factors and comorbid conditions that affect asthma
Pharmacologic therapy
The goals of therapy are to achieve asthma control by reducing impairment and risk:
- Prevent chronic and troublesome symptoms (e.g., coughing or breathlessness in the daytime, in the night, or after exertion)
- Require infrequent use (≤2 days a week) of inhaled SABA for quick relief of symptoms
- Maintain (near) “normal” pulmonary function
- Maintain normal activity levels (including exercise and other physical activity and attendance at work or school)
- Meet patients’ and families’ expectations of and satisfaction with asthma care
- Prevent recurrent exacerbations of asthma and minimize the need for ED visits or hospitalizations
- Prevent progressive loss of lung function; for children, prevent reduced lung growth
- Provide optimal pharmacotherapy with minimal or no adverse effects
Patients’ detailed recall of symptoms decreases over time; therefore, the clinician may choose to assess over a 2-week, 3-week, or 4-week recall period. Symptom assessment for periods longer than 4 weeks should reflect more global symptom assessment, such as inquiring whether the patient’s asthma has been better or worse since the last visit and inquiring whether the patient has encountered any particular difficulties during specific seasons or events.
Low FEV1 is associated with increased risk of severe asthma exacerbations. Regular monitoring of pulmonary function is particularly important for asthma patients who do not perceive their symptoms until airflow obstruction is severe. There is no readily available method of detecting the “poor perceivers.” The literature reports that patients who had a near-fatal asthma exacerbation, as well as older patients, are more likely to have poor perception of airflow obstruction.
Long-term control medications
Corticosteroids: Block late-phase reaction to allergen, reduce airway hyperresponsiveness, and inhibit inflammatory cell migration and activation. They are the most potent and effective anti-inflammatory medication currently available. ICSs are used in the long-term control of asthma. Short courses of oral systemic corticosteroids are often used to gain prompt control of the disease when initiating long-term therapy; long-term oral systemic corticosteroid is used for severe persistent asthma.
Cromolyn sodium and nedocromil: Stabilize mast cells and interfere with chloride channel function. They are used as alternative, but not preferred, medication for the treatment of mild persistent asthma. They can also be used as preventive treatment prior to exercise or unavoidable exposure to known allergens.
Immunomodulators: Omalizumab (anti-IgE) is a monoclonal antibody that prevents binding of IgE to the high-affinity receptors on basophils and mast cells. Omalizumab is used as adjunctive therapy for patients ≥12 years of age who have allergies and severe persistent asthma. Clinicians who administer omalizumab should be prepared and equipped to identify and treat anaphylaxis that may occur.
Leukotriene modifiers: (Montelukast, pranlukast, zafirlukast, and zileuton). Target a single group of inflammatory mediators by either blocking the leukotriene receptor or reducing the activity of enzymes required for leukotriene synthesis. Two LTRAs are available—montelukast (for patients >1 year of age) and zafirlukast (for patients ≥7 years of age). The 5-lipoxygenase pathway inhibitor zileuton is available for patients ≥12 years of age; liver function monitoring is essential. LTRAs are alternative, but not preferred, therapy for the treatment of mild persistent asthma (Step 2 care). LTRAs can also be used as adjunctive therapy with ICSs, but for youths ≥12 years of age and adults. Zileuton can be used as alternative but not preferred adjunctive therapy in adults.
LABAs: Salmeterol and formoterol after a single dose administration have at least 12 hours duration of bronchodilation. Because of their slower onset of action, the uses of LABA for the treatment of acute symptoms or exacerbations is not currently recommended or is monotherapy for long-term control of asthma.
- LABAs are used in combination with ICSs for long-term control and prevention of symptoms in moderate or severe persistent asthma (step 3 care or higher in children ≥5 years of age and adults)
- Of the adjunctive therapies available, LABA is the preferred therapy to combine with ICS in youths ≥12 years of age and adults
- The beneficial effects of LABA in combination therapy for the great majority of patients who require more therapy than low-dose ICS alone to control asthma (i.e., require step 3 care or higher) should be weighed against the increased risk of severe exacerbations, although uncommon, associated with the daily use of LABAs (see discussion in text).
For patients ≥5 years of age who have moderate persistent asthma or asthma inadequately controlled on low-dose ICS, the option to increase the ICS dose should be given equal weight to the option of adding LABA. For patients ≥5 years of age who have severe persistent asthma or asthma inadequately controlled on step 3 care, the combination of LABA and ICS is the preferred therapy.
LABA may be used before exercise, but duration of action does not exceed 5 hours with chronic regular use. Frequent and chronic use of LABA for EIB is discouraged, because this use may disguise poorly controlled persistent asthma.
Methylxanthines: Sustained-release theophylline is a mild to moderate bronchodilator used as alternative, not preferred, adjunctive therapy with ICS (Evidence A). Theophylline may have mild anti-inflammatory effects. Monitoring of serum theophylline concentration is essential.
Quick-relief medications
Anticholinergics: Inhibit muscarinic cholinergic receptors and reduce intrinsic vagal tone of the airway. Ipratropium bromide provides additive benefit to SABA in moderate-to-severe asthma exacerbations. May be used as an alternative bronchodilator for patients who do not tolerate SABA (Evidence D).
SABAs: Albuterol, levalbuterol, and pirbuterol are bronchodilators that relax smooth muscle that have become constricted as a result of environmental stimuli. Therapy of choice for relief of acute symptoms and prevention of EIB. SABAs will provide rapid relief of symptoms, although do not target underlying inflammation associated with asthma.
Systemic corticosteroids: Although not short acting, oral systemic corticosteroids are used for moderate and severe exacerbations as adjunct to SABAs to speed recovery and prevent recurrence of exacerbations.
Other treatments
Allergen Immunotherapy
Allergen injection immunotherapy is effective in allergic asthma as well as in allergic rhinoconjunctivitis and has been shown to lead to highly significant improvements in symptoms, reduction in rescue medication, and improvements in both allergen specific and non-specific bronchial hyperresponsiveness. Immunotherapy is particularly effective in seasonal asthma, although less effective in perennial asthma. Bronchial asthma is a risk-factor for systemic reactions to immunotherapy and should not be considered in poorly controlled asthmatics. Allergy management is superimposed upon other treatment modalities for long-term control at all levels of asthma. Concurrent upper airway disease, eg, allergic rhinitis, sinusitis, should be treated, and the total dose of inhaled corticosteroids must be monitored.
Biological treatment: Omalizumab (monoclonal anti-IgE antibody) may be considered as adjunctive therapy in step 5 or 6 care for patients who have allergies and severe persistent asthma that is inadequately controlled with the combination of high-dose ICS and LABA. Omalizumab is effective in reducing asthma exacerbations and hospitalizations in patients with increased levels of total IgE. It is recommended for use in moderate to severe asthma patients as an adjunctive therapy to inhaled steroids and during steroid tapering, in patients with steroid-resistant asthma, and in patients who need to reduce or withdraw their inhaled steroids.
Bronchial thermoplasty (BT) is a novel therapy for patients with severe asthma. Using radio frequency thermal energy, it aims to reduce the airway smooth muscle mass. Several clinical trials have demonstrated improvements in asthma-related quality of life and a reduction in the number of exacerbations following treatment with BT. In addition, recent data has demonstrated the long-term safety of the procedure as well as sustained improvements in rates of asthma exacerbations, reduction in health care utilization, and improved quality of life.
In the past 10 years, there have been substantial advances in the understanding of asthma genetics, airway biology, and immune cell signaling. These advances have led to the development of small molecule therapeutics and biologic agents that may improve asthma care in the future. Several new classes of asthma drugs—including ultra-long acting β agonists and modulators of the interleukin 4 (IL-4), IL-5, IL-13, and IL-17 pathways—have been evaluated in randomized controlled trials. Other new drug classes—including dissociated corticosteroids, CXC chemokine receptor 2 antagonists, toll-like receptor 9 agonists, and tyrosine kinase inhibitors—remain in earlier phases of development.
Other co-morbid conditions treatment
In all patients, symptomatic therapies are also given, to be used on an as needed basis. The goal in all of these patients is to tailor the medicines and their doses to control the level of the disease, always trying for optimal control with the lowest effective dose of medications. At least half of US adults with asthma have at least 1 other chronic condition. Having asthma and other chronic conditions are associated with poorer asthma outcomes. Several studies considered the relationship between asthma and other specific chronic conditions; results of these studies indicated that having depression or anxiety and/or panic disorder is associated with an increased risk of developing a new asthma diagnosis and with poorer asthma outcomes. In addition, results of these studies indicated that having asthma is associated with an increased risk of developing a new depression or anxiety and/or panic disorder diagnosis.
Current NIH guidelines recommend that all patient who have had an asthma-related hospitalization should be evaluated by asthma specialist. In doing so, patients may likely improve quality of life and decrease asthma-related morbidity and mortality
Bibliography
- National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, Full report 2007
- Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. Lötvall J1, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. J Allergy Clin Immunol. 2011 Feb;127(2):355-60. doi: 10.1016/j.jaci.2010.11.037
- Asthma in the elderly: what we know and what we have yet to know. Anahí Yáñez, Sang-Hoen Cho, Joan B Soriano, Lanny J Rosenwasser, Gustavo J Rodrigo, Klaus F Rabe, Stephen Peters, Akio Niimi, Dennis K Ledford, Rohit Katial, Leonardo M Fabbri, Juan C Celedón, Giorgio Walter Canonica, Paula Busse, Louis-Phillippe Boulet, Carlos E Baena-Cagnani, Qutayba Hamid, Claus Bachert, Ruby Pawankar, Stephen T Holgate. World Allergy Organ J. 2014; 7(1): 8. Published online 2014 May 30. doi: 10.1186/1939-4551-7-8
Perinatal and Early Childhood Environmental Factors Influencing Allergic Asthma Immunopathogenesis. Jonathan M. Gaffin, Watcharoot Kanchongkittiphon, Wanda Phipatanakul. Int Immunopharmacol. 2014 September; 22(1): 21–30. Published online 2014 June 19. doi: 10.1016/j.intimp.2014.06.005 PMCID: PMC4119505 - Global strategy for asthma management and prevention: GINA executive summary.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M et al. Eur Respir J 31(1):143-78. 2008 DOI:10.1183/09031936.00138707 PMID: 18166595
- The Genetics of Asthma and Allergic Disease: A 21st Century Perspective
Carole Ober, Tsung-Chieh Yao. Immunol Rev. Author manuscript; available in PMC 2012 July 1. Published in final edited form as: Immunol Rev. 2011 July; 242(1): 1030.doi: 10.1111/j.1600-065X.2011.01029.x PMCID: PMC3151648 - International Consensus On (ICON) Pediatric Asthma
N. G. Papadopoulos, H. Arakawa, K.-H. Carlsen, A. Custovic, J. Gern, R. Lemanske, P. Le Souef, M. Makela, G. Roberts, G. Wong, H. Zar, C. A. Akdis, L. B. Bacharier, E. Baraldi, H. P. van Bever, J. de Blic, A. Boner, W. Burks, T. B. Casale, J. A. Castro-Rodriguez, Y. Z. Chen, Y. M. El-Gamal, M. L. Everard, T. Frischer, M. Geller, J. Gereda, D. Y. Goh, T. W. Guilbert, G. Hedlin, P. W. Heymann, S. J. Hong, E. M. Hossny, J. L. Huang, D. J. Jackson, J. C. de Jongste, O. Kalayci, N. Khaled, S. Kling, P. Kuna, S. Lau, D. K. Ledford, S. I. Lee, A. H. Liu, R. F. Lockey, K. Lodrup-Carlsen, J. Lotvall, A. Morikawa, A. Nieto, H. Paramesh, R. Pawankar, P. Pohunek, J. Pongracic, D. Price, C. Robertson, N. Rosario, L. J. Rossenwasser, P. D. Sly, R. Stein, S. Stick, S. Szefler, L. M. Taussig, E. Valovirta, P. Vichyanond, D. Wallace, E. Weinberg, G. Wennergren, J. Wildhaber, R. S. Zeiger. Allergy. Author manuscript; available in PMC 2015 May 26.Published in final edited form as: Allergy. 2012 August; 67(8): 976–997. Published online 2012 June 15. doi: 10.1111/j.1398-9995.2012.02865.x PMCID: PMC4442800
Additional references:
Buttaro, T.M., Polgar-Bailey, P., Sandberg-Cook, J. & Trybulski, J. (2017). Primary care: A collaborative practice (5th ed.) St. Louis, MO: Elsevier.
https://www.nhlbi.nih.gov/files/docs/guidelines/asthma_qrg.pdf
https://www.frontiersin.org/articles/10.3389/fimmu.2019.00821/full
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4818235/

Phone Number
WAO Secretariat
Suite 1100
Milwaukee, WI 53202-3823
USA
