Diagnostic Approach to the Adult with Suspected Immune Deficiency - Chinratanapisit S, Sriaroon P, Sleasman JW, Condino-Neto A (Updated 2021)
Diagnostic Approach to the Adult with Suspected Immune Deficiency
Updated: March 2021
Updated: June 2015
Originally Posted: December 2008
Updated by:
Antonio Condino-Neto, MD, PhD
Professor of Immunology and Experimental Medicine
Department of Immunology, Institute of Biomedical Sciences
University of São Paulo
São Paulo, SP. Brazil
Original Authors:
Sasawan Chinratanapisit, M.D.
Division of Allergy and Immunology, Dept of Pediatrics
Bhumibol Adulyadej Hospital Bangkok, Thailand
Panida Sriaroon, M.D.
Division of Allergy, Immunology, and Rheumatology
University of South Florida, Dept. of Pediatrics
Saint Petersburg, FL, USA
John W. Sleasman, M.D.
Professor and Chief
Division of Allergy and Immunology
Duke University School of Medicine
Durham, NC, USA
1.0 Disease Definition
Primary and secondary immunodeficiency disorders are a diverse group of illnesses resulting from one or more abnormalities of the immune system. The clinical manifestations include increased susceptibility to infection and an increased risk for autoimmune disease, malignancy, allergy, and autoinflammatory disorders. Primary immune deficiency (PID) diseases are a group of serious disorders arising from an intrinsic defect in the immune system, generally the result of a genetic disease that can be traced directly to a particular immune pathway, currently, primary defects over 400 genes. In contrast, secondary immune deficiencies stem from impairment of the immune response through other mechanisms, such as an infection, metabolic derangement, malignancy, toxins, immunosuppressant therapies, with the immune defect being a secondary manifestation. Although the possibility of immunodeficiency should be considered in any individual with recurrent infections, it is also important to consider nonimmune conditions as a cause. Such disorders include circulatory abnormalities leading to stasis or cellular ischemia, as can occur in sickle cell disease, diabetes or heart failure; obstructive lung conditions such as chronic obstructive pulmonary disease, ciliary dyskinesia, and cystic fibrosis; or ureteral stenosis, resulting in defective pathogen clearance; and breaches in the integument and mucosal surfaces, as is seen in erythema multiform or burns, epidermolysis bullosa, severe inflammatory bowel disease, allowing entry to opportunistic organisms and infection. Each of these is associated with infection by specific pathogens and results in characteristic clinical manifestations.
Secondary immune dysfunctions are far more common in adults than PID. Common causes include malnutrition, HIV/AIDS, malignancy, immune suppressive drugs, and toxin exposure. Malignancy can directly suppress B-cell function, as is seen in chronic lymphocytic leukemia and lymphomas. Malignancy can also directly cause bone marrow failure, resulting in neutropenia and impaired T-cell function.
Metabolic disorders such as diabetes, uremia and liver failure are often associated with severe, life threatening infections due to impairment of the cellular functions of lymphoid and myeloid cells. Protein-losing enteropathies, burns and nephrosis lead to loss of soluble factors, such as immunoglobulin and complement, thereby increasing the risk for sepsis and peritonitis. Autoimmune diseases, especially those associated with immune complexes such as systemic lupus erythematosus (SLE), often result in secondary complement deficiency due to chronic consumption that exceeds hepatic synthesis. In large case series from Thailand and Taiwan, anticytokine autoantibodies against interferon-gamma, associated with multiple opportunistic infections and were associated with an adult-onset immunodeficiency. Autoantibodies against type I interferons are associated with severe COVID19 outcome, and autoantibodies against IL-17 area associated with chronic mucocutaneous candidiasis in AIRE deficiency, and autoantibodies agains IL-6 are associated with staphylococcus sepsis. Transient activation and depletion of complement can also occur with sepsis, viremia, burns and trauma. In the diagnostic approach to an adult with recurrent infections and suspected immune deficiency, these nonimmune and secondary causes need to be considered. The characteristics and clinical features of the categories of primary and secondary immune disorders in adults are listed in Table 1.
2.0 Classification and Clinical Presentation
Although less frequent than secondary immune disorders, PID can be quite common. For example, in some ethnic populations, such as Scandinavians, mannose-binding lectin deficiency has a prevalence of 5%-7%. Selective immunoglobulin (Ig)A deficiency occurs in as many as 1 in 300 individuals. These common PIDs are usually of low clinical relevance, whereas other PIDs can result in life-threatening infections. The International Union of Immunological Societies (IUIS) Expert Committee on PIDs recently reported on the updated classification of PIDs. As in recent classifications, eight major groups of PID have been included
- Immunodeficiencies affecting cellular and humoral immunity
- Combined immunodeficiencies with associated or syndromic features
- Predominantly antibody deficiencies
- Diseases of immune dysregulation
- Congenital defects of phagocyte number, function, or both
- Defects in intrinsic and innate immunity
- Autoinflammatory disorders
- Complement deficiencies
- Bone marrow failure
- Phenocopies of inborn errors of immunity
PIDs are a group of over 400 monogenic diseases, which evolve with recurrent infections, autoimmunity, immune dysregulation, autoinflammation, malignancy, and allergy phenotypes. This a ever growing number of diseases that were classified according to phenotypes, genotypes or both.
2.1 Antibody Deficiencies are the most common cause of PID, accounting for over half of all cases. Clinical manifestations are primarily recurrent bacterial sinopulmonary infections, including pneumonia and sinusitis, although bacterial sepsis and meningitis have been frequently reported. Infections with Haemophilus influenzae, Streptococcus pneumoniae and other encapsulated bacterial organisms are most common. Manifestations are not limited to sinopulmonary infections, as chronic Giardia enteritis, gastrointestinal lymphoid hyperplasia, polymyositis, autoimmune cytopenias, and chronic arthritis also occur. The most common clinically significant antibody deficiency disorder in adults is common variable immune deficiency (CVID). CVID is often associated with recurrent bacterial pneumonia, often with bronchiectasis; persistent gastrointestinal symptoms, suggestive of inflammatory bowel disease or celiac disease; and autoimmunity. As many as 20% of patients with CVID develop granulomas in the lungs, liver and spleen that can be confused with sarcoidosis. While a genetic cause cannot be identified in the majority of patients with clinical features of CVID, there are an increasing number of identificable conditions that can be linked to newly identified genetic disorders that have implications for the long term prognosis and management of CVID. For example, patients with PIK3CD deficiency have Ig profiles consistent with CVID as well as recurrent sinopulmonary infection but also have decreased herpes virus driven lympho-proliferative disease, low naïve CD4 T cells and inverted CD4/CD8 T cell ratios. Other signs of a specific genetic disorders in patients with CVID include intestinal amyloidosis (NLRP12 deficiency), CNS granulomas and enteropathy (CTLA4 deficiency). A comprehensive clinical and laboratory evaluation along with a detailed family history should be part of the evaluation of all patients with CVID. Other antibody deficiencies seen in adults that have similar clinical presentations to CVID include IgG subclass deficiency, selective IgA deficiency, functional antibody deficiency, B- cell chronic lymphocytic leukemia, and protein-losing enteropathies.
2.2. Cellular Immune Deficiency. Clinical symptoms resulting from a primary defect in cellular immunity or combined cellular and antibody immunity are more rare in adults than in children; instead, secondary immune deficiencies associated with HIV infection or drugs targeting T-cell function predominate. Commonly encountered infections consist of chronic mucocutaneous candidiasis, persistent infections with cytomegalovirus and other herpes viruses such as simplex or zoster, Pneumocystis jiroveci pneumonia and infections with atypical mycobacteria species. Defective cellular immunity increases the risk for malignancy, particularly B-cell lymphomas or lymphoproliferative diseases, driven by Epstein-Barr virus.
2.3. Phagocytic Cell Deficiency. Defective phagocytic cell function and neutropenia syndromes are commonly associated with recurrent infections of the soft tissues, including cellulitis, lymphadenitis and osteomyelitis. Abscesses and granulomas caused by fungal pathogens within the liver, lung and spleen are also manifested. Many times, the clinical presentation will consist solely of fever of unknown origin due to occult deep-seated infection. Oral and periodontal disease is common, including chronic necrotizing gingivitis, oral ulcers and dental abscesses. Poor or delayed wound healing is often observed. Systemic infections with catalase-positive organisms such as Staphylococcus aureus, Serratia, Aspergillus, Burkholderia cepacia and Nocardia are the hallmark of chronic granulomatous disease which is defective in oxidative burst capacity. Rare late presenting forms of phagocytic disorders include Hyper-IgE syndrome, autosomal recessive (p47phox) chronic granulomatous disease, IL-12 and interferon gamma receptor (IFNgR) pathway defects. Defects in the IL-12 and interferon gamma receptor (IFNgR) pathway result in chronic infections with atypical mycobacteria species. Hyper-IgE syndrome is due to a mutation in STAT3 resulting in severe eczema, soft tissue infections, and staphylococcal pneumonia. Secondary causes of neutrophil disorders include myelodysplastic syndromes, malignancy, myeloablative chemotherapy, and autoimmune neutropenia.
2.4. Complement Deficiency. Hereditary deficiencies in the complement system have highly variable clinical manifestations depending on the particular component of the complement cascade affected. Impairment of the regulatory proteins involved in C1 inhibitor produces clinical symptoms that include episodic nonpruritic angioedema of the airway, soft tissues and gastrointestinal tract. Individuals with defects in the early or classical complement components have an increased risk for bacterial infection and commonly develop autoimmune diseases such as SLE, rheumatoid arthritis and glomerulonephritis. Patients with defects in the terminal components of the complement cascade often develop chronic or recurrent infections, especially meningitis, with Neisseria species and also have increased risk for autoimmunity. Most complement disorders are inherited in an autosomal-dominant or co-dominant pattern, making a family history helpful in the diagnosis.
2.5. Severe COVID 19. A recent study of over 650 individuals who developed severe COVID-19 found that approximately 3.5% of patients harbored germline loss-of-function variants in genes previously found to be important for host defense against influenza or other viral infections (bi-allelic loss of function mutations of IRF7 or IFNAR1, heterozygous mutations in TLR3, TICAM1, TBK1, or IRF3) due to the key role of these genes in the type 1 IFN signaling pathway. An accompanying study found that, strikingly, approximately 10% of patients with severe COVID-19 have high levels of neutralizing autoantibodies (autoAbs) against type 1 IFNs in their serum. The impact of these autoAbs was evidenced by the inability to detect type 1 IFN in serum from these patients, and their capacity to prevent anti-viral immune responses in vitro.
3.0 Evaluation for Suspected Immunodeficiency
Evaluation of suspected PID in the adult patient should be based on the clinical findings associated with the categories of immune deficiency diseases. The diagnosis should be directed toward primary and secondary etiologies that are consistent with the clinical presentation and pattern of infections. Therefore, the nature of the infecting organism, the history and clinical presentation, and systematic use of the laboratory and diagnostic procedures can be utilized to identify the nature of the immune defect. The results will assist in identifying which patients need to be referred to tertiary centers for definitive diagnosis and treatment or can be managed by the primary practicing allergist and immunologist.
3.1 History is an important initial step in the evaluation process. Unusual or severe complications of common infections are often seen in patients with immune deficiency. For example, empyema associated with bacterial pneumonia suggests antibody deficiency. Hepatic or splenic granuloma caused by S. aureus suggests chronic granulomatous disease. The need to treat infection with prolonged or repeated use of antibiotics is common in patients with immune deficiency. The medical history should include a complete family history to determine if there is a pattern of inheritance consistent with known PID, such as autosomal dominant, X-linked, or autosomal recessive patterns. When taking the family history, the physician should inquire about consanguinity and recurrent or severe infections among family members. Associated noninfectious features of immune deficiency, such as premature loss of dentition or poor or delayed wound healing, should be investigated. Recurrent herpes zoster infections or persistent cutaneous warts are common in patients with cellular immune deficiencies, whereas a history of recurrent aphthous ulcers may be the only clue that phagocyte function is defective.
3.2 Physical examination also provides clues to the nature of immune deficiency. For example, wasting and unexplained weight loss is common in both antibody and cellular immune deficiencies, although any chronic inflammatory condition can be associated with abnormal catabolic states. Scarred tympanic membranes or chronic perforation due to recurrent otitis media is commonly seen in patients with antibody deficiencies. Coarse facial features and severe eczema suggest a hyper-IgE syndrome, whereas vitiligo or alopecia areata are associated with mucocutaneous candidiasis with autoimmune polyglandular disease. Hepatosplenomegaly and lymphadenopathy are frequently found in patients with CVID and cellular immune deficiency. Cutaneous fungal infections suggest defects in cellular immunity. Furuncles and soft tissue abscesses are seen in phagocytic disorders. Ocular telangiectasia in association with cerebellar ataxia is the hallmark of ataxia telangiectasia, which leads to progressive combined immunodeficiency. Chronic inflammatory arthritis is seen in antibody and complement deficiencies.
3.3 Diagnostic testing should focus on distinguishing between non-immune, secondary, and PID diseases. Evaluation of recurrent sinopulmonary infections should include, where necessary, a detailed radiographic assessment of the sinuses and high resolution imaging of the lungs to detect occult bronchiectasis, hilar adenopathy, and granulomas. Interpretations of the findings should be in the context of other laboratory and clinical findings. For example, granulomas in the lungs, liver or spleen can be seen in chronic granulamatous disease, CVID or sarcoidosis. In patients with recurrent pneumonia, ciliary biopsy and sweat testing for late-presentation cystic fibrosis should be considered. Neutrophil counts and splenic function should be assessed in patients with bacterial sepsis. All forms of neutropenia should be further evaluated with a bone marrow aspirate to assess for occult malignancy or myelodysplasia. The complete metabolic profile of all patients with severe or recurrent infections should be evaluated to assess hepatic, renal and endocrine function; a urinalysis should also be performed to detect occult proteinuria.
3.4 Laboratory assessment of immunity consists of step-by-step analysis that correlates the clinical manifestations with the specific suspected immune disorder.
In order to optimize the use of the clinical laboratory, testing should first focus on quantitative and qualitative screening evaluations, with more costly and complex testing used to pinpoint the precise nature of the disorder and confirm the suspected diagnosis. A summary of the screening tests and studies to confirm the diagnosis is provided in Table 2. Screening tests for defects in antibody production include quantitative serum immunoglobulins, in which total IgG, IgA, and IgM can be precisely measured. The predominant immune globulin in the blood is IgG, normally ranging from 800 to 1500 mg/dL with a slight decline after the age 60. Selective IgA deficiency is defined as serum levels < 10 mg/dL. Typical IgM levels in adults range from 70 to 130 mg/dL. Isolated selective IgM deficiency (< 10 mg/dL) is extremely rare whereas elevated IgM is indicative of hyper IgM syndrome, infection or autoimmunity. Elevations in multiple isotypes may reflect polyclonal B cell activation as in HIV or other viral infections such as EBV or CMV, sarcoidosis, or SLE. Elevations in a single isotype should be evaluated by serum protein electrophoresis in order to exclude multiple myeloma, Waldenstrom's macroglobulinemia, or primary systemic amyloidosis. As many as 1% of normal adults of age 50 develop benign monoclonal gammopathy. Specific antibody titers measured prior to and 30 days after immunization is an accepted means to measure functional immunity to T-dependent (tetanus and diphtheria) and T-independent (different serotypes of pneumococcal polysaccharide) antigens. In more than 90% of healthy individuals usual postimmunization protective response to T-dependent antigens such as tetanus and diphtheria exceeds 1.0 IU/ml. The response to T-independent antigens is generally measured by pre and post titers to different serotypes within the pneumococcal polysaccharide vaccine. The protective level of antibody following the pneumococcal polysaccharide vaccine is poorly defined, however, the ACAAI and AAAAI practice parameters define a normal response in adults to be titers ³ 1.3 µg/ml or a four-fold rise in at least 70% of the immunizing serotypes.
Elevation in total serum IgE can be helpful in suspected patients who have IgE mediated allergic rhinosinusitis, allergic asthma, and other allergic diseases from those who have antibody defects. In patients with low total IgG or defective functional immunity, measurement of IgG subclasses is helpful in pinpointing the diagnosis. However, the significance of IgG subclass deficiency in the presence of normal antibody responses to protein and polysaccharide antigen is not known. Low or absent IgG4 is present in up to 5% of healthy adults and its deficiency is not considered to be clinically relevant. Enumeration of B cells using flow cytometry can differentiate late-onset congenital agammaglobulinemia where CD20+ or CD19+ B cells are low or absent from CVID where total B cell numbers are generally normal.
Screening laboratory tests to evaluate cellular immunity include total lymphocyte count, T- and B-cell enumeration using flow cytometry and HIV antibody testing. HIV is the most common secondary immune deficiency among adults. In healthy individuals, CD3-positive T cells make up 60%-70% of the total lymphocyte count. These cells are sub-divided into CD4 T cells or CD8 T cells in a typical ratio that is greater than 1.0. Inverted CD4 to CD8 T ratios with low total CD4 T cell numbers are seen in AIDS, some CVID subtypes, and idiopathic CD4 T-cell lymphopenia. Functional assessment of cellular immunity includes delayed type hypersensitivity (DTH) skin testing to recall antigens and mitogens and antigen-induced lymphocyte proliferation, which are in vitro measurements of lymphocyte function computed as a stimulation index. For example, the antigen-specific proliferation response and DTH to Candida is low in mucocutaneous candidiasis. Genetically characterized, combined immune deficiency diseases due to defects in purine salvage pathways or T-cell signaling rarely have their initial clinical presentation in adolescents and adults. Clinical manifestations of these disorders usually occur in infancy and are fatal if untreated.
A complete blood count to enumerate neutrophils, other granulocytes and monocytes can be used as an initial test for phagocyte deficiencies. Persistent leukocytosis over 25,000 cells/µl suggests a possible leukocyte adhesion deficiency that can be confirmed on the basis of low expression of adhesion molecules such as CD11b and CD18. Detection of rare variants of chronic granulomatous disease reported in adults and adolescents can be done by flow cytometry analysis using dihydrorhodamine dye or oxidative respiratory burst assays which have largely replaced the nitroblue tetrazolium assay as the best assessment of phagocyte function. The different genetic variants of chronic granulomatous disease, 91phox, p22 and p47, have recognizable patterns of fluorescence that can help confirm the diagnosis.
Screening tests for the complement system include total C3, total C4, total serum hemolytic complement (CH50) and alternative pathway (AH50). Together, these four tests can be used to differentiate defects in the classical, alternative or terminal complement components. The C3 and C4 levels are helpful in determining if there is ongoing complement consumption, as is seen in immune complex or autoimmune diseases. In these conditions, multiple complement components are depressed, whereas in isolated inherited deficiencies, the absence of a single component results in abnormal function of the entire pathway. A low or totally depressed CH50 with normal AH50 is characteristic of defects in the classical pathway (examples are C1, C2 and C4 deficiency). A low or absent AH50 with normal CH50 is typical of a defect in the alternative pathway (such as properdin deficiency). Low or absent AH50 and CH50 (C5 through C9) is characteristic of defects in the terminal complement components that make up the membrane attack complex. Abnormal results should be followed with factor specific assays to identify the specific deficient component. In mannose-binding lectin deficiency, the AH50, CH50, C3, and C4 are normal, but the mannose-binding lectin level is low. The diagnosis can be confirmed and further characterized by genotyping for the various alleles associated with these disorders.
4.0 Management
The management of PID depends on which component of the immune system is impaired and whether function can be restored. In cases involving severe defects in cellular immunity bone marrow transplantation may be the only treatment available to reverse the condition. In older patients, this procedure generally requires pre-ablation with chemotherapy and immune suppression, carrying an inherent risk for poor outcome. Gene therapy may become an alternative for the treatment of single gene defects in the near future. In contrast, patients with antibody deficiency IgG can be replaced with monthly intravenous gammaglobulin or weekly infusions of subcutaneous IgG. This therapy is effective in controlling sinopulmonary infections and their complications. The use of gammaglobulin replacement therapy should be reserved for those individuals who show laboratory evidence of a defect in humoral immunity, have recurrent infections, fail conservative therapy with antibiotics, and have no other causes for their recurrent infections.
Cellular immune deficiency, particularly those associated with low CD4 T cell counts, should receive prophylaxis for opportunistic infections. Current recommendations are that patients with CD4 T cell counts of less than 200 cells/µl should have prophylaxis with trimethoprim/ sulfamethoxazole for Pneumocystis jiroveci pneumonia, and those with CD4 T cell counts of less than 50 cells/µl should have prophylaxis for atypical mycobacterium infections. Patients with defective phagocytic cell function should receive prophylaxis for Aspergillus. Patients with chronic granulomatous disease benefit from gamma interferon replacement therapy. All PIDs are chronic and life-long conditions that require close clinical monitoring and aggressive interventions to optimize health and quality of life.
Table 1: Characteristics of Immunodeficiency Disorders in Adults
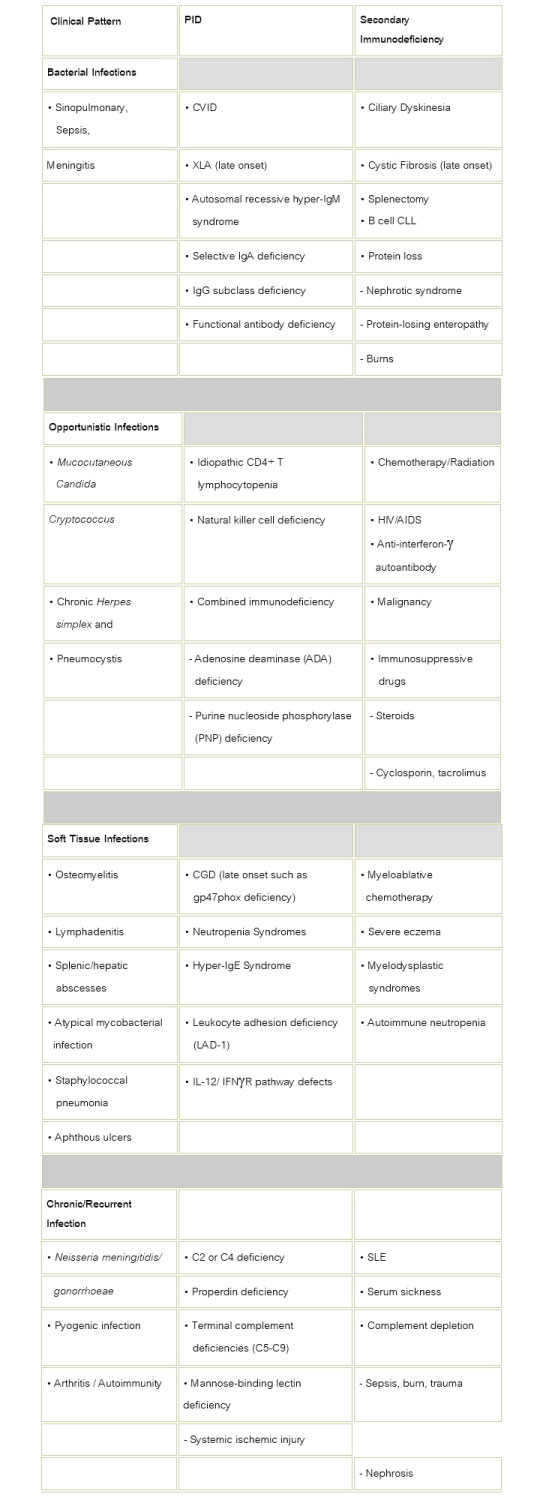
Table 2: Laboratory Values for Evaluation of T Cell or B Cell Immune Deficiency
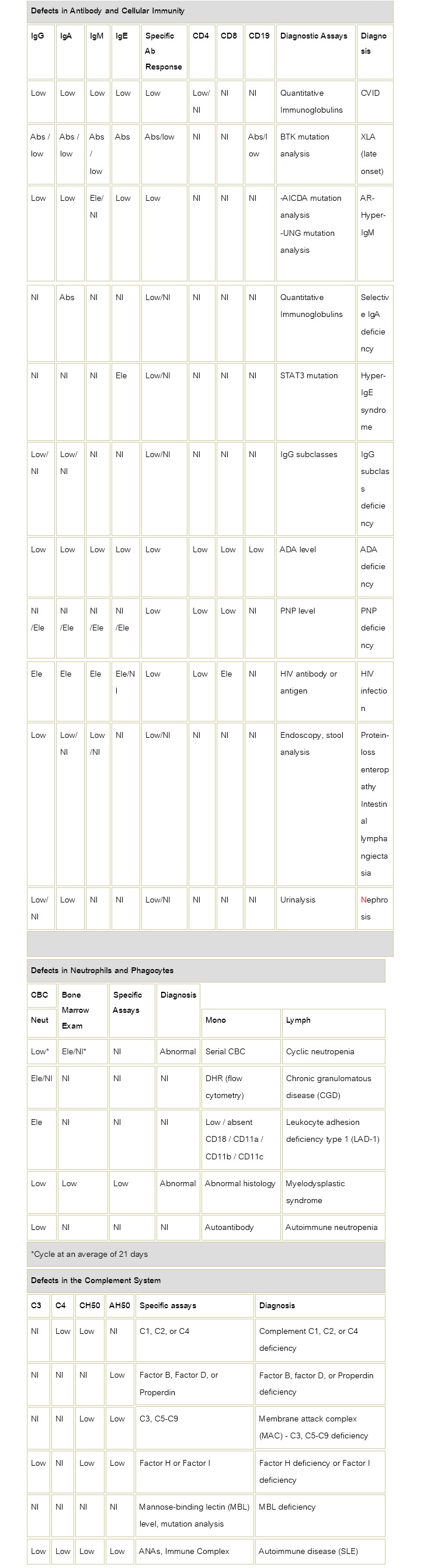
Abs: Absent Nl: Normal, Ele: Elevated, CVID: common variable immune deficiency, BTK: Bruton tyrosine kinase, XLA: X-linked agammaglobulinemia, CLL; Chronic Lymphocytic Leukemia, AID: Activation-induced cytidine deaminase, ADA: Adenosine deaminase, PNP: Purine nucleoside phosphorylase, IL-12: Interleukin-12, IFNγR; Interferon gamma receptor, CBC: Complete blood cell count, DHR: Dihydrorhodamine dye, SLE: Systemic lupus erythematosus, AR-Hyper-IgM: Autosomal Recessive Hyper-IgM, STAT 3: Signal transducer and activator of transcription 3, ANAs: Antinuclear antibodies
Bibliography
- Sleasman JW, Virella G. Diagnosis of immunodeficiency diseases. In: Virella G. Medical Immunology. 6th ed. New York, NY:Informa Healthcare; 2007: 397-407
- Orange JS, Hossny EM, et al. Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology: J Allergy Clin Immunol. 2006 April;117(4):525-553
- Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency: Ann Allergy Asthma Immunol 2005; 94(5 Suppl 1):S1-S63
- Ballow M, O'Neil KM. Approach to the patient with recurrent infections. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, eds. Middleton's Allergy Principles and Practice. 6th ed. Philadelphia, PA: Mosby; 2003:1043-1072
- Wen L, Atkinson JP, Giclas PC. Clinical and laboratory evaluation of complement deficiency: J Allergy Clin Immunol. 2004 April;113(4):585-593
- Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections: J Allergy Clin Immunol. 2004 April;113(4):620-626,
- Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Picard C, Puck J, Torgerson TR, Casanova JL, Sullivan KE. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020 Jan;40(1):24-64. doi: 10.1007/s10875-019-00737-x
- Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, Klein C, Morio T, Oksenhendler E, Picard C, Puel A, Puck J, Seppänen MRJ, Somech R, Su HC, Sullivan KE, Torgerson TR, Meyts I. The Ever-Increasing Array of Novel Inborn Errors of Immunity: an Interim Update by the IUIS Committee. J Clin Immunol. 2021 Apr;41(3):666-679. doi: 10.1007/s10875-021-00980-1.
- Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020; 370(6515):eabd4585. doi: 10.1126/science.abd4585
- Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570.
